pH Basics
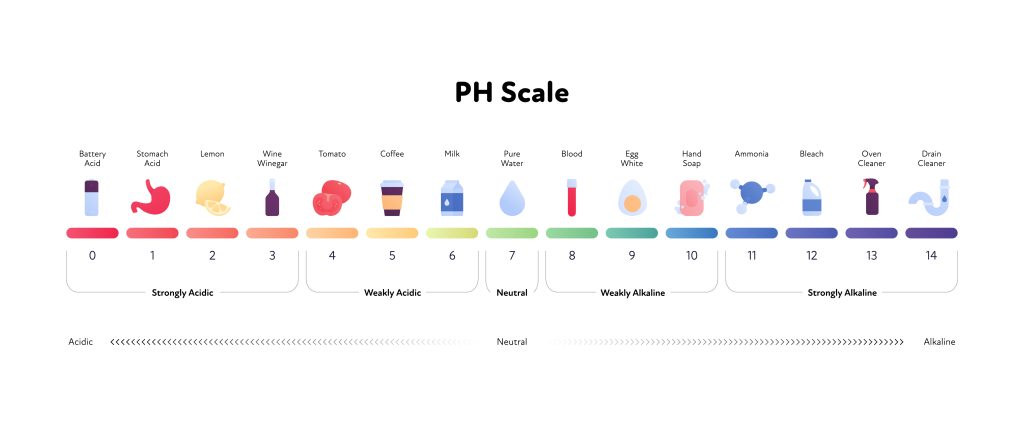
You may remember from high school chemistry that the pH scale ranges from zero (extremely acidic) to 14 (extremely basic or alkaline). Pure water has a neutral pH of 7. The pH scale is logarithmic, meaning that a pH of 6 is ten times more acidic than a pH of 7.
What is a “Healthy” pH?
In natural health circles there has been a recent push to “alkalinize”. Like many trends, there is a kernel of truth to this push — a diet that is high in vegetables, fruits, and legumes tends to result in metabolic waste (also called “ash”) that is alkaline, whereas a diet high in meat products and processed foods tends to result in metabolic waste products that are acidic. Of course most people could benefit from eating more vegetables and fewer processed foods, so adopting a diet that results in alkaline ash probably represents a healthy step.
“Alkalinizing” the entire body is not possible, however. The normal pH of blood ranges from 7.35-7.45. This level is tightly controlled by dozens of metabolic processes — if the pH of blood deviates far outside this range, the consequences are serious or even deadly. Changes in diet may alter the pH of urine, but significantly changing the pH of the blood with diet is not possible (and even it if were, it would be not be desirable).
Is “Alkaline Water” Good for You?
As indicated in the scale above, the pH of pure H2O is 7. You may have noticed store shelves filling with alkaline water products, some of them with a pH as high as 9.5 or 10, with the implication that there are health benefits to drinking high pH water. Both physiologically and clinically, this is not the case, however.
A healthy stomach has an extremely low (acidic) pH of approximately 1.5. An highly acidic environment in the stomach is crucial for health — it is one of the first steps to the digestion of food, it kills the bacteria that is present on all foods, and it activates the co-factors that are necessary for the absorption of certain nutrients (among other things). Drinking high pH water quenches the acid that is normally present in the stomach and can result in overgrowth of pathogenic bacteria in the small intestine and compromised absorption of nutrients. Clinically I have see a number of patients who have developed whole-body water retention and swelling that resolved when they stopped drinking alkaline water. I have also seen patients rapidly develop large kidney stones on alkaline water and experience worsening of pre-existing allergies. For these reasons,
I strongly urge against alkaline water.
pH and Skin Health
Healthy skin has a slightly acidic pH of approximately 5.2-5.7. An appropriate “acid mantle” is important for maintaining the population of the beneficial bacteria that exists on the skin, as well as appropriate skin moisture and immune function. As you can see on the scale above, however, soap has an alkaline pH of approximately 10. Daily use of soap represents a strain on the skin that can result in dryness and accelerated skin aging. Daily use of soap is particularly apt to cause problems for people with pre-existing skin issues such as acne, eczema, psoriasis, skin allergies, severe body odor, etc.
In a state of health, the female genital area has an even lower pH than the skin on the rest of the body — the beneficial bacteria in this area and the immune system functions most effectively at a pH of 3.8-5.0. Women who suffer from frequent and/or severe bacterial or yeast infections of the vagina and/or bacterial infections of the bladder often benefit a great deal from replacing high pH soap-based products with a slightly acidic cleanser. In some women, switching to a lower pH cleansing product is all that is needed to restore health of the genital area. Other women with established infection require initial treatment using a product like
Yin-Care mixed with a small quantity of vinegar, followed with ongoing use of a low-pH cleansing product.
Low pH Cleansing Products
Fortunately low pH cleansing products have become more readily available in recent years. Here are Amazon links to a few products that I have tried personally or my patients have tried (products labeled as facial cleansers can be used in the genital area and/or on the body):


